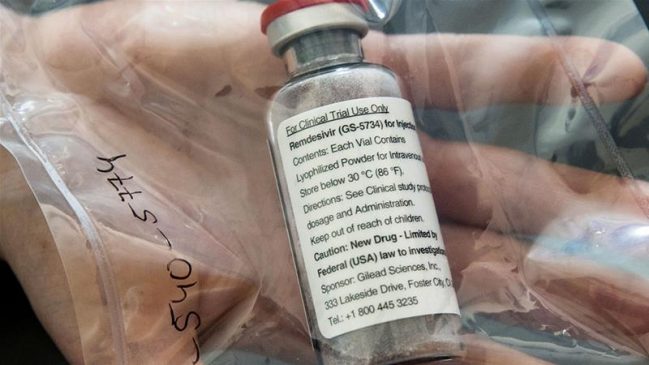
Pharmaceutical company Gilead Sciences on Wednesday reported potentially encouraging results from a trial for a drug that’s being tested as a treatment for the coronavirus.
Gilead said that the antiviral drug Remdesivir produced “similar improvement” in patients over a 10-day treatment plan compared with a five-day treatment plan – and that it recorded “no new safety” issues among hospitalized patients who “well-tolerated” the treatment in the study.
“The study demonstrates the potential for some patients to be treated with a 5-day regimen, which could significantly expand the number of patients who could be treated with our current supply of Remdesivir,” said Merdad Parsey, Chief Medical Officer of Gilead Sciences, in a statement. “This is particularly important in the setting of a pandemic, to help hospitals and healthcare workers treat more patients in urgent need of care.”

The initial results released Wednesday come after a report two weeks ago by the medical site STAT suggested potentially positive results in one of its Remdesivir trials.
Remdesivir is one of numerous drugs under development to treat or prevent the coronavirus. Clinical trials are conducted to ensure safety and efficacy, and there’s no guarantee Gilead’s initial results will lead to a commercially available treatment. But medical professionals have had high hopes for Remdesivir since the coronavirus pandemic began.
Gilead emphasized that Remdesivir “is not yet licensed or approved anywhere globally and has not yet been demonstrated to be safe or effective for the treatment of COVID-19.”
This particular study involved patients suffering from pneumonia and reduced oxygen levels who did not require ventilation when the study began.
Gilead reported that it took 10 days for 50% of the patients in the five-day treatment group to show clinical improvement, compared with 11 days for patients in the 10-day treatment group. Source: Yahoonews